Recent change in childhood immunisation schedule
In recent years, there have been a number of changes to the PCV schedule, mainly due to the success of the pneumococcal vaccine programme.
High uptake of the PCV has resulted in very low levels of disease caused by pneumococcal bacteria, and in 2018 the UK Joint Committee on Vaccination and Immunisation (JCVI) agreed that one dose of the vaccine plus a booster should continue to provide good protection for children and the community.
See the minutes from the JCVI meeting.
One dose plus a booster is still the routine. From July 2025 in the UK the first dose is being given at 16 weeks, with the booster at 12-13 months.
How well does the pneumococcal conjugate vaccine (PCV) work?
It is estimated that in the first 11 years of the pneumococcal vaccine programme (2006-07 to 2016-17), the vaccine prevented nearly 40,000 cases of invasive pneumococcal disease, and about 2000 deaths.
The original version of the PCV (Prevenar7) was introduced in 2006. This vaccine protected against seven of the types of bacteria and resulted in a big reduction in the number of cases of pneumococcal disease in babies caused by these seven types. However, there was an increase in the number of cases caused by other types of pneumococcal bacteria. Six strains in particular were identified as causing most of the new cases of pneumococcal disease.
In 2010, the PCV vaccine was changed to Prevenar13 as it covers more strains of pneumococcal bacteria. Research in 2011 by the UK Health Protection Agency (now The UK Health Security Agency - UKHSA) showed a big reduction in serious disease caused by the additional six types of pneumococcal bacteria protected against by the new vaccine.
Another study by Public Health England (now UKHSA) published in 2015 confirmed that eight years of PCV use in England and Wales had reduced the overall incidence of invasive pneumococcal disease by more than 50%. The PCV has a herd immunity effect. Vaccination of babies and toddlers has reduced the amount of disease in the whole population, because infants and children are no longer carrying so many pneumococcal bacteria and spreading them around.
However, the 2015 research found that other strains of pneumococcal bacteria were starting to become more common, and could partly replace the strains that are disappearing, especially in non-vaccinated older individuals. The UKHSA has continued to monitor this trend. It has found that strains not covered by the PCV have increased, and are causing more cases of pneumococcal disease (over 4000 cases in 2015-16, compared with about 2000 cases in 2010-11). Recent reports can be found here .
At the moment the disease caused by these strains is generally less severe and less likely to be fatal. There is still a very substantial reduction in disease in young children but non-vaccine strains have replaced the reductions in disease in older age groups to some extent. The UKHSA is continuing to monitor the situation. New PCV products with an increased number of types of bacteria (PCV15 and PCV20) have been licensed and other products are in development globally.
In 2022, PCV15, which protects against the PCV13 pneumococcal serotypes as well as serotypes 22F and 33F, was licensed for use from 6 weeks of age. The JCVI agreed that the current evidence indicated it could be used in a 1+1 schedule.
PCV20 protects against 20 pneumococcal serotypes and is currently licensed for use in adults. These vaccines are not included in the UK national immunisation programme at the moment, although the JCVI/UKHSA is continuously looking at how to optimise the UK vaccination schedule.
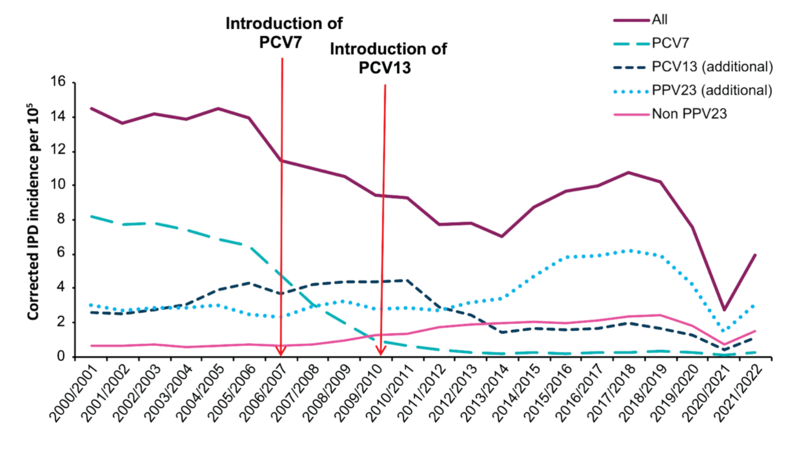
In January 2020, the UK moved to a new infant schedule, with the first dose given at 12 weeks, followed by a booster at 12-13 months. Because of the COVID-19 pandemic lockdown and subsequent restrictions since March 2020, cases of pneumococcal disease decreased by 65% across all age groups in 2020/21. As restrictions were eased, Invasive Pneumococcal Disease (IPD) incidence gradually increased, initially in young children followed by older children and adults. See the diagram below.
Source: The Green Book
This 2020 change has been followed by the most recent change – the shift from 12 to 16 weeks - so that babies don’t have too many injections at one time as the MenB vaccine was brought forward to 12 weeks to provide earlier protection again this more prevalent disease.
How well does the pneumococcal polysaccharide vaccine (PPV) work?
The PPV is designed to protect against 23 common types of pneumococcus bacteria. Most healthy adults develop a good antibody response to a single dose by the third week following immunisation. Children younger than two years of age show poor antibody responses to immunisation with PPV23 and there is no evidence of effectiveness of PPV23 in this age group.
In the UK, the PPV has been recommended for risk groups since 1992 and for all people aged 65 years and over since 2003. PPV had moderate short-term effectiveness of 41% against pneumococcal disease caused by the vaccine serotypes in adults aged 65 years and over during the first two years after vaccination, with vaccine effectiveness being higher among healthy individuals compared to those with underlying medical conditions. See the full research paper here.
There is some evidence of the PPV offering protection against non-bacteraemia pneumococcal pneumonia.
The length of protection offered by the PPV in risk groups and in older adults is variable and dependent upon the type of bacteria. Antibody levels after immunisation usually begin to wane (lessen) after about five years but may decline more rapidly in asplenic (without a spleen) patients and children with nephrotic syndrome (a disorder in the kidneys).
Source: The Green Book
JCVI recommendation
The Joint Committee on Vaccination and Immunisation (JCVI) recommends including PCV20 for all adults in risk groups as PCV20 is likely to prevent more disease than PPV23. This is because of the larger health benefit of higher-valency PCVs, and the way pneumonia spreads in the community is changing over time. The protection given by PCV20 may lessen (wane) at a slower rate compared to the PPV23 vaccine.
Read the JCVI minutes here.