The flu vaccine works better in some years than others (see the inactivated flu vaccine page for full information about how the vaccine works).
US studies of the H1N1 (‘Swine Flu’) pandemic in 2009 found that pregnant women were four times as likely to develop serious illness and up to five times as likely to be admitted to hospital, compared with the general population. As a result of the evidence from this pandemic, pregnant women were added to the list of groups considered to be at higher risk from seasonal flu.
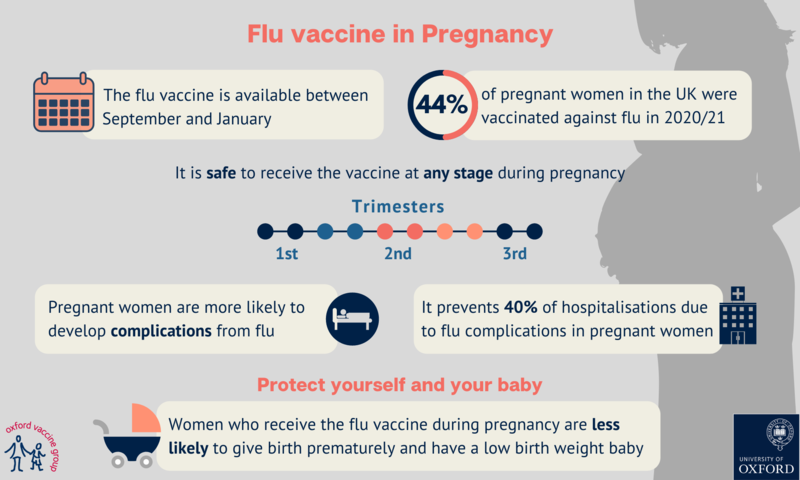
In the UK between 2009 and 2012, flu was the cause of death for 36 women who died during pregnancy or shortly afterwards. It is estimated that half of these deaths could have been prevented by flu vaccination. See the 2014 summary report from MBRRACE-UK (Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK).
Recent research covering almost 20,000 pregnant women over six years in the United States, Australia, Israel, and Canada, showed that the flu vaccine provided a 40% reduction in hospitalisations from flu. The PREVENT (Pregnancy Influenza Vaccine Effectiveness Network) study looked at data between 2010 and 2016 to identify flu-related hospital admissions (see PREVENT research paper).
Studies have shown that women who have been vaccinated against flu are less likely to give birth prematurely, and less likely to have a low-birthweight baby (see the results of a Canadian study). Other studies have shown that women who have the flu vaccine while pregnant are less likely to experience stillbirth (see the results of an Australian study).
Flu vaccination in pregnancy also means that flu antibodies are transferred through the placenta to the baby. This gives the baby some protection against flu for the first few months of life.
The inactivated flu vaccine does not contain any live flu viruses and cannot give you flu.