|
Seasonal influenza is a very common and highly infectious virus. It can be much more severe than the common cold and may result in two or three days in bed, leading to missed work and school days. For some at-risk groups (especially frail elderly people and individuals with certain health conditions), flu can be very dangerous. Flu complications may lead to more than ten thousand hospital stays and an average of 600 deaths in the UK every year but this can vary. In 2012, there were 83 deaths from influenza in England and Wales, whereas in 2018, there were 1598 deaths. The flu virus constantly changes, so different strains circulate yearly. The World Health Organization monitors the virus throughout the world and advises which 3 or 4 strains should be covered by an annual flu vaccine. Flu vaccination is offered annually because the previous year's vaccine may not provide protection against the strains that circulate in a new season and because immunity needs to be boosted each year even for the same strain. In the UK, the flu vaccine is available each year from late September or early October onwards. Eligible people are recommended to get their flu vaccine in the autumn or early winter before outbreaks begin. It takes up to two weeks after vaccination for protection to develop. In the 2020-21 season, more than 19 million adults and children were vaccinated against flu. This year’s autumn flu vaccine programmes will start earlier than planned in England as a precautionary measure following the identification of a new COVID-19 variant. Vaccinations are now set to start on 11 September 2023 in England with adult care home residents and those most at risk to receive vaccines first. In the 2023-24 season, three different types of inactivated flu vaccine are available - QIVe, QIVc, and QIVr. As well as the live nasal flu vaccine – LAIV – which is offered to children over 2 years of age and adolescents. A separate version of the vaccine, aQIV, is also available for older people. This has added ‘adjuvant’ to make the vaccine more effective in older people. All these vaccines used in the UK are currently ‘quadrivalent’ and protect against four strains of the flu. See the table in ‘more information’ below to find out which vaccine is recommended for each age group. 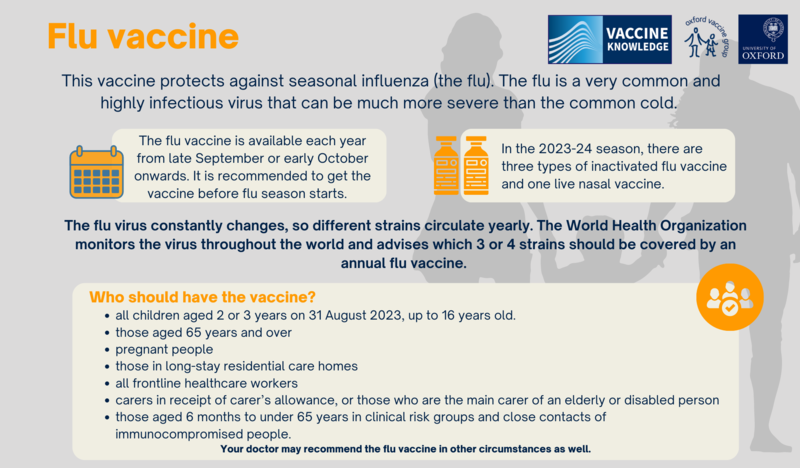
|
Flu vaccine
Vaccine
|
In the UK the groups of people recommended for vaccination against flu is subject to change annually. In the 2023/24 flu season, the following people are eligible to receive the flu vaccine free of charge:
Your doctor may recommend the flu vaccine in other circumstances as well. Inactivated flu vaccines are only licensed from 6 months of age, and the LAIV flu vaccine from 2 years. Ideally, people should have received their vaccination by the end of December, although there can still be some benefit to offering vaccination until the end of March as flu can continue to circulate. |
|
All vaccines go through rigorous testing and regulatory processes that can take up to 15 years to ensure they are safe and effective. Vaccines can cause side effects like all medicines, but not everyone experiences them. Inactivated flu vaccine The inactivated flu vaccine does not contain the live virus and cannot cause flu. Flu vaccines have a very good safety record. The most reported side effects of flu vaccines are:
Live flu vaccine The live flu vaccine contains a weakened version of the flu virus. There is no evidence that healthy unvaccinated people can catch the flu from the nasal flu spray. Either from airborne spray droplets in the room where the vaccine is given or from vaccinated individuals ‘shedding’ the virus. The most reported side effects are: Very common (affecting more than 1 in 10 people)
Common (affecting up to 1 in 10 people):
Uncommon (affecting up to 1 in 100 people):
There are several different flu vaccines available each year. For more information on side effects, ask for the patient information leaflet for the vaccine you are offered. Additional information about vaccine side effects, anaphylaxis and adverse reactions can be found here. As with any vaccine, medicine or food, there is a very small chance of a severe allergic reaction (anaphylaxis). Anaphylaxis is different from less severe allergic reactions because it causes life-threatening breathing and/or circulation problems. It is always serious but can be treated with adrenaline. In the UK between 1997 and 2003 there were a total of 130 reports of anaphylaxis following ALL immunisations, but all of these people survived. Around 117 million doses of vaccines were given in the UK during this period, making the overall rate around 1 in 900,000. Depending on the cause of the reaction, and following expert guidance, the person may be able to have vaccinations in the future. |
|
Several different brands of flu vaccine are used in the UK each flu season. For full information on ingredients, ask for the patient information leaflet for the vaccine you are offered or look the brand name up on the electronic Medicines Compendium (eMC). Inactivated flu vaccines used in the UK often contain very small amounts of the following ingredients:
Some inactivated flu vaccines usually contain very small amounts of egg proteins (ovalbumin), as the virus is often grown on hens’ eggs. Egg-produced vaccines with a low ovalbumin content have a lower risk for egg-allergic people. People who are allergic to eggs should ask their doctor for specialist advice. The main vaccine recommended for those over 65 years of age, aQIV is produced using eggs but alternatives are available (QIVr and QIVc) for egg-allergic older adults. However, the injected vaccines which have been prioritised for use in the UK in younger adults, such as QIVc and QIVr are not grown in egg cells and so do not carry this risk. See more information on egg proteins in vaccines. The UK Health Security Agency (UKHSA) produce an information sheet showing the ovalbumin content of flu vaccines in the current flu season. aQIV, the vaccine recommended for people aged 65 and over is produced using hen’s eggs and contains a small amount of an adjuvant called MF59. Adjuvants are added to help strengthen and lengthen the immune response to the vaccine. The main ingredient in MF59 is squalene oil, a naturally occurring oil found in humans, plants, and animals. The nasal flu spray, LAIV, contains very small amounts of these added ingredients:
Very sensitive tests have shown that no DNA from pigs can be found in the LAIV vaccine.
|
|
There is strong evidence that pregnant women have a much higher risk of serious illness because of flu, compared with the general population. The risks are highest in the last three months of pregnancy. Vaccination against the flu reduces the risk of complications caused by the virus for women and babies. Serious complications of flu include bronchitis, pneumonia, septic shock (a severe and life-threatening infection of the whole body), meningitis and encephalitis (inflammation of the brain). There is also strong evidence that catching the flu during pregnancy has an effect on the unborn baby. Babies born to women who have had flu are up to four times more likely to be born prematurely and to have a low birth weight. This may be because flu infection produces an inflammatory response in the body which can trigger premature labour. Flu in pregnancy can even lead to stillbirth or death in the first week of life. 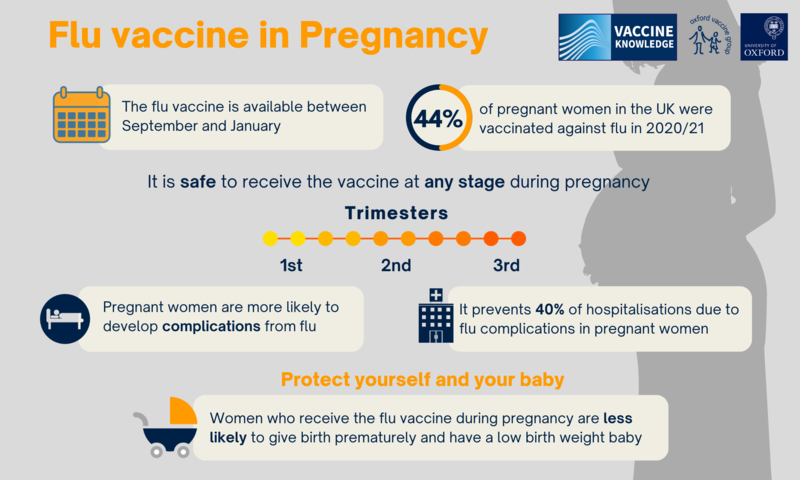
Click here for an accessible text version of this infographic
A 2019 study with 20,000 pregnant women over six years in the United States, Australia, Israel, and Canada, showed that the flu vaccine provided a 40% reduction in hospitalisations from flu. A US study published in 2017 looked at the effects of flu vaccination in the first three months of pregnancy. They compared 52,000 babies who had been exposed to the flu vaccine in the first three months of pregnancy with over 370,000 babies who had not been exposed to the flu vaccine. The study showed that having the flu vaccine in early pregnancy was not associated with an increased risk of birth defects. |
Recommended flu vaccinesIn the 2023-24 season, three different types of inactivated flu vaccine are available - QIVe, QIVc, and QIVr. QIV is a shortened version of the 'quadrivalent influenza vaccine’ – this means the influenza vaccines are quadrivalent and protect you against four different strains of influenza. The three types of QIV are ‘e’,’c’, or ‘r’. ‘E' is a shorter version of egg-based, ‘c’ is a shorter version of cell-based, and ‘r’ is short for recombinant (made using an insect cell line). There is also one other type of QIVe available for older people, this is called aQIV. The ‘a’ is short for ‘adjuvanted’ and this means an ‘adjuvant’ has been added to make the vaccine more effective in older people. Finally, the nasal flu vaccine – LAIV is available for children aged 2-18 years. LAIV is short for ‘live attenuated influenza vaccine’, which means the vaccine contains whole viruses which have been ‘weakened’ to create an immune response without causing disease in healthy people. For more information about types of vaccines, visit types of vaccines, or see the table below to find out which influenza vaccine is recommended for each age group.
Live flu vaccineVaccinated children shed the virus for a few days after vaccination through sneezing or coughing. However, the vaccine virus is weakened, so it is much less able to spread from person to person than flu viruses that normally spread. The amount of virus that children shed is normally below the levels needed to pass on the infection to others. The virus does not survive for long outside the body. It is not necessary for children to be off school during the period when the vaccine is being given. The only exception is the very small number of children who are extremely immunocompromised. These children are usually advised not to attend school anyway, because of the higher risk of being in contact with infections that spread in school. The nasal flu spray should not be given to anyone with a weakened immune system. This includes people who:
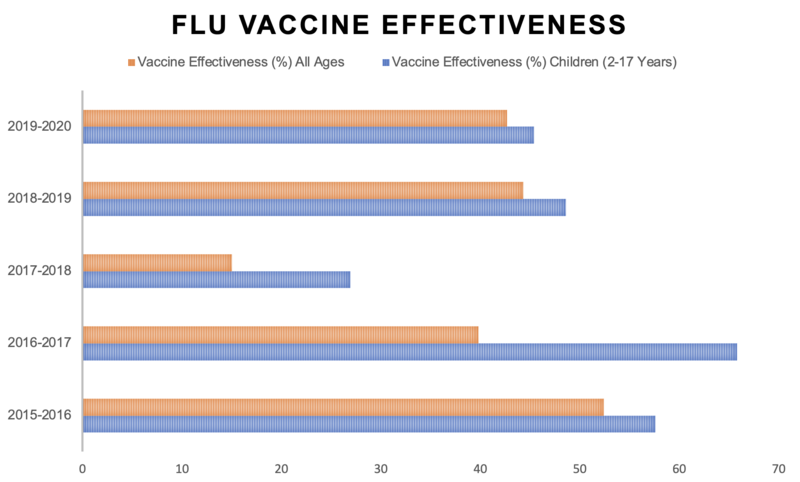
This is because the weakened viruses in the vaccine could replicate too much and cause infection. Children who have been vaccinated with the nasal spray should avoid close contact with people who have very severely weakened immune systems for about two weeks following vaccination. If it is not possible for a child to avoid contact with someone who is severely immunosuppressed, e.g because they live in the same house, the child should not receive the nasal flu vaccine. It may be possible for them to have the inactivated flu vaccine instead. How effective is the NHS flu immunisation programme?The flu vaccine works better in some years than others. Across all age groups including children, the flu vaccine prevented between 15 to 52% of flu cases between 2015 - 2020 For the breakdowns of each year, please consult Public Health England's data on the effectiveness of flu vaccines in the 2015-16 season, 2016-17 season, 2017-18 season, 2018-19 season and 2019-20 season. Due to low levels of circulating influenza in the 2020-21 flu season, it has not been possible to determine vaccine effectiveness for this time period.
Click here for an accessible text version of this graph
Protection from the flu virus varies for different age groups. In children aged 2-17, the flu vaccine prevented 66% of flu cases in 2016-17, 27% of flu cases in 2017-18, and 49% of flu cases in 2018-19. The people taking part in these studies were patients who had visited their general practitioner (GP) during the study period with flu-like symptoms. However, in the over-65 age group, the inactivated flu vaccine worked less well than it did in other groups. In 2016-17, the data suggest that the inactivated flu vaccine did not work at all in people aged over 65, whilst in 2017-2018 it resulted in slightly better results in that age group. To address this problem of lower effectiveness in older people, an inactivated vaccine containing an adjuvant was introduced for the 2018-19 season. This is a substance that strengthens and lengthens the immune response. It has resulted in better prevention of flu in people aged 65 or over in flu seasons since 2018-19. The adjuvanted vaccine is still recommended for this age group in the 2022-23 season. It is not understood why flu vaccines do not work so well in older adults. However, this reinforces the importance of vaccinating children and healthcare workers, both of whom can help to stop the spread of flu to older adults. As well as protecting others, you will also be protecting yourself against getting the flu, or from experiencing severe symptoms.
Why do we need the flu vaccine every year?Having a flu vaccine every year is important because the flu virus is variable and changes over time. Each year there are different strains, and a new vaccine must be prepared to deal with them. Vaccination from previous years is not likely to protect people against current strains of flu. 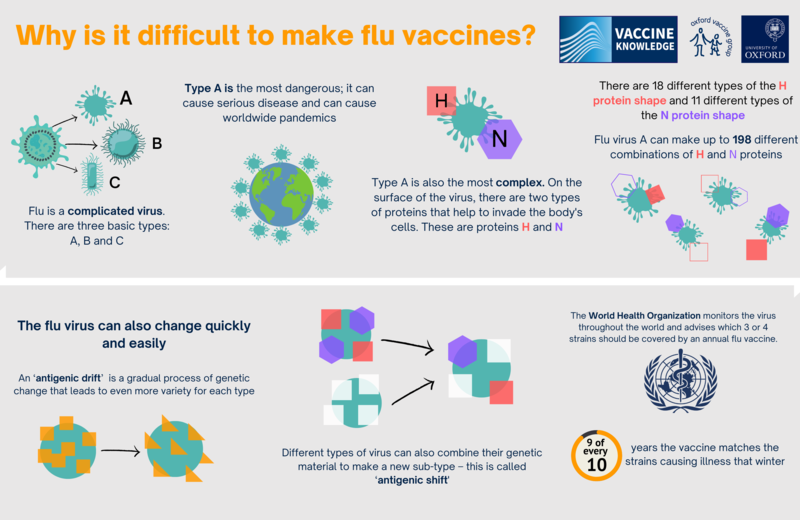
Click here for an accessible text version of this infographic
Each year’s flu vaccine is made to give the best protection against the strains of flu that are expected to circulate (spread) in the coming season. The trivalent vaccine protects against three of the flu virus strains which are most likely to be around. The quadrivalent vaccine protects against four flu virus strains. However, decisions about what to put in the flu vaccine must be made six months before the flu season starts. Every February, the World Health Organisation (WHO) reviews the types of flu that have been circulating in all parts of the world and decides which ones will go into the vaccine for the following autumn. This allows time for the vaccine to be made – but it also gives the flu virus time to change before vaccination starts in the autumn. This means that sometimes the flu vaccine may not be a good match for all the strains of flu that are circulating. Read more about the WHO recommendations for the 2022-23 season. Researchers are investigating ways to create a flu vaccine that protects against the many different varieties of flu. If they are successful, it will mean that people will only need a single flu vaccine to give them lifelong protection, instead of having a yearly vaccine. However, it will be several years before we find out if it is possible to do this. The virus for the QIVe flu vaccine is usually grown in hen’s eggs. This is a slow process and can lead to something called ‘egg adaptation’. The flu virus strain starts to adapt to the conditions in the egg, leading to changes in the virus. This is another reason that the flu vaccine may not always match the circulating strains of flu. Since 2018-19, a new vaccine has been developed using cell culture instead of eggs for flu seasons. This vaccine is less likely to adapt as it has been developed using cells that are very similar to human cells. Cell-based vaccines are now recommended for people over two years unless the recommended vaccine is not suitable or unavailable. For example, due to porcine gelatine content. |



